BRAIN STEM RETICULAR FORMATION
The general characteristics of reticular regions may be summarized as follows. They tend to be ill-defined collections of neurones and fibres with diffuse connections. Their conduction paths are difficult to define, complex and often polysynaptic, and they have ascending and descending components that are partly crossed and uncrossed. Their components subserve somatic and visceral functions. They include distinct chemoarchitectonic nuclear groups, including clusters of serotoninergic neurones (group B cells), which synthesize the indolamine 5-hydroxytryptamine (serotonin); cholinergic neurones (group Ch cells), which contain acetyltransferase, the enzyme which catalyses the synthesis of acetylcholine; and three catecholaminergic groups composed of noradrenergic (group A), adrenergic (group C), and dopaminergic (group A) neurones, which synthesize noradrenaline (norepinephrine), adrenaline (epinephrine) and dopamine respectively as neurotransmitters.
Studies with the Golgi technique show that few brain stem reticular neurones are classic Golgi type II neurones (i.e. with short axons that branch locally). In contrast, they have long dendrites that spread across the long axis of the brain stem in transverse sheets. These radiating dendrites may spread into 50% of the cross-sectional area of their half of the brain stem, and they are intersected by, and may synapse with, a complex of ascending and descending fibres. Many axons of the reticular neurones ascend or descend, or bifurcate to do both. They travel far, perhaps through the whole brain stem and often beyond. As an example, a bifurcating axon from a cell in the magnocellular medullary nucleus may project rostrally into the upper medulla, pons, midbrain tegmentum, subthalamus, hypothalamus, dorsal thalamus, septum, limbic system and neocortex, while its descending branch innervates the reticular core of the lower medulla and may reach the cervical spinal intermediate grey matter (laminae V and VI). Many reticular neurones have unidirectional, shorter axons, which synapse with the radiating dendrites of innumerable other neurones en route, and give off collaterals, which synapse with cells in 'specific' brain stem nuclei or cortical formations, such as the cerebellum. Multitudes of afferent fibres converging on individual neurones and their myriad synapses and destinations provide the structural basis for the polymodal responses elicited by experiment, and also for such terms as 'diffuse', 'non-specific' polysynaptic systems.
A contrasting dendritic form is also found, in which dendrites are short, sinuous or curved, branch profusely and pursue re-entrant courses at the perimeter of a nuclear group, defining a boundary between it and its environs. Neurones with an intermediate dendritic complexity occur in and near such nuclei and vary in density in much of the remaining reticular formation. In different zones, the proportion of different sizes of neuronal somata varies. Some regions contain only small to intermediate multipolar cells ('parvocellular' regions). However, there are a few areas where these mingle with large multipolar neurones in 'gigantocellular' or 'magnocellular' nuclei.
In general terms the reticular formation is a continuous core that traverses the whole brain stem, and is continuous below with the reticular intermediate spinal grey laminae. It is divisible, on the basis of cytoarchitectonic, chemoarchitectonic and functional criteria, into three bilateral longitudinal columns: median; medial, containing mostly large reticular neurones; and lateral, containing mostly small to intermediate neurones (Fig. 19.22).
MEDIAN COLUMN OF RETICULAR NUCLEI
The median column of reticular nuclei extends throughout the medulla, pons and midbrain and contains neurones that are largely aggregated in bilateral, vertical sheets, blended in the midline and occupying the paramedian zones. Collectively they are called the nuclei of the raphe, or raphe nuclei (Fig. 19.22). Many neurones in raphe nuclei are serotoninergic and are grouped into nine clusters, B1-9. The raphe pallidus nucleus and associated raphe obscurus nucleus lie in the upper two-thirds of the medulla and cross the pontomedullary junction. The raphe magnus nucleus, corresponding to many B3 neurones, partly overlaps them, and ascends into the pons. Above it is the pontine raphe nucleus, which is formed by the cell group B5. Also located in the pons is the central superior raphe nucleus, which contains parts of cell groups B6 and B8. The dorsal (rostral) raphe nucleus, approximating to cell group B7, ascends - it expands, then narrows, through much of the midbrain.The serotoninergic raphe system ramifies extensively throughout the entire CNS. Although many of these fibres may be diffusely distributed, recent work has revealed substantial preferential innervation by discrete parts of the system. For example, whereas the central superior raphe nucleus projects divergently to all areas of the cortex, different neurones in the dorsal raphe nucleus not only project specifically to circumscribed regions of the frontal, parietal and occipital cortex, but also to functionally related regions of the cerebellar cortex. Similarly, the caudate nucleus and putamen receive a preferential input from the dorsal raphe nucleus, whereas the hippocampus, septum and hypothalamus are innervated mainly by cells in the central superior mesencephalic raphe nucleus.
All raphe nuclei provide mainly serotoninergic descending projections, which terminate in the brain stem and spinal cord. Brain stem connections are multiple and complex. For example, the dorsal raphe nucleus, in addition to sending a large number of fibres to the locus coeruleus, projects to the dorsal tegmental nucleus and most of the rhombencephalic reticular formation, together with the central superior, pontine raphe and raphe magnus nuclei.
Raphe spinal serotoninergic axons originate mainly from neurones in the raphe magnus, pallidus and obscurus nuclei. They project as ventral, dorsal and intermediate spinal tracts in the ventral and lateral funiculi, and terminate respectively in the ventral horns and laminae I, II and V of the dorsal horns of all segments, and in the thoracolumbar intermediolateral sympathetic and sacral parasympathetic preganglionic cell columns. The dorsal raphe spinal projections function as a pain-control pathway that descends from the mesencephalic pain-control centre, which is located in the periaqueductal grey matter, dorsal raphe and cuneiform nuclei. The intermediate raphe spinal projection is inhibitory, and, in part, modulates central sympathetic control of cardiovascular function. The ventral raphe spinal system excites ventral horn cells and could function to enhance motor responses to nociceptive stimuli and to promote the flight and fight response.
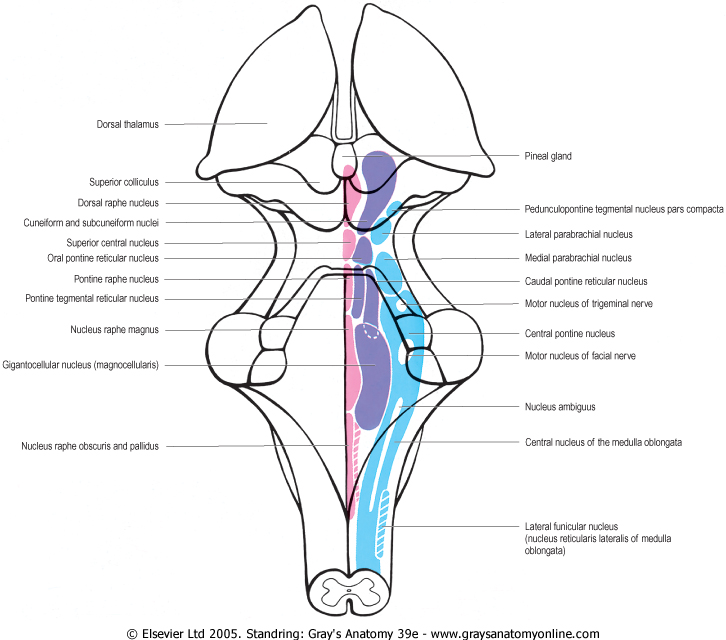
Figure 19.22 An outline of the human brain stem (black) extending from the caudal end of the medulla to the dorsal thalami. Note the margins of the rhomboid fossa, the lateral angles of which indicate the pontomedullary junction. Note also the profiles of the transected surfaces of the cerebellar penducles, the colliculi and pineal gland. The principal nuclear derivatives of the brain stem reticular formation are indicated in approximate outline. Those from the median and paramedian nuclear column are in magenta; medial column derivatives are purple, lateral column derivatives blue. In reality, considerable overlap of the nuclear profiles would be present when the third dimension is considered. A number of 'non-reticular' nuclei are also included.
Principally, the mesencephalic serotoninergic raphe system is reciprocally interconnected rostrally with the limbic system, septum, prefrontal cortex and hypothalamus. Efferents ascend and form a large ventral and a diminutive dorsal pathway. Both originate from neurones in the dorsal and central superior raphe nuclei. The raphe magnus nucleus also contributes to the dorsal ascending serotoninergic pathway, which is at first incorporated into the dorsal longitudinal fasciculus (of Schütz). A few fibres terminate in the central mesencephalic grey matter and posterior hypothalamus, but most continue into the medial forebrain bundle and merge with the axons of the ventral pathway, which are distributed to the same targets. The fibres of the ventral ascending serotoninergic pathway exit the ventral aspect of the mesencephalic raphe nuclei, and then course rostrally through the ventral tegmentum from where fibres pass to the ventral tegmental area, substantia nigra and interpeduncular nucleus. A large number of fibres then enter the habenulointerpeduncular tract and run rostrally to innervate the habenular nucleus, intralaminar, midline, anterior, ventral and lateral dorsal thalamic nuclei, and the lateral geniculate body. The ventral ascending serotoninergic pathway enters the median forebrain bundle in the lateral hypothalamic area and splits to pass medially and laterally. The fibres in the medial tract terminate in the mammillary body, dorsomedial, ventromedial, infundibular, anterior and lateral hypothalamic, medial and lateral preoptic and suprachiasmatic nuclei. Those in the lateral tract take the ansa peduncularis-ventral amygdalofugal path to the amygdala, striatum and caudal neocortex. The medial forebrain bundle carries the remaining ventral ascending serotoninergic axons into the medullary stria, stria terminalis, fornix, diagonal band, external capsule, cingulate fasciculus and medial olfactory stria, to terminate in all the structures that these systems interconnect.
Major afferents into the mesencephalic raphe nuclei include those from the interpeduncular nucleus linking the limbic and serotoninergic systems; the lateral habenular nucleus linking the septum, preoptic hypothalamus and prefrontal cortex via the habenulointerpeduncular tract and the medial forebrain bundle; and the pontine central grey matter.
The ascending raphe system probably functions to moderate forebrain activities, particularly limbic, septal and hypothalamic activities. Recent demonstrations of specific connectivity suggest that it exerts precise, as well as tonal, control.
MEDIAL COLUMN OF RETICULAR NUCLEI
The medial column of reticular nuclei is composed predominantly of neurones of medium size, although very large neurones are found in some regions, and most have processes orientated in the transverse plane (Fig. 19.22). In the lower medulla the column is indistinct, and is perhaps represented by a thin lamina lateral to the raphe nuclei. However, in the upper medulla it expands into the medullary gigantocellular (magnocellular) nucleus, which lies ventrolateral to the hypoglossal nucleus, ventral to the vagal nuclei and dorsal to the inferior olivary complex. Ascending further, the column continues as the pontine gigantocellular (magnocellular) nucleus, which lies medially in the tegmentum. Its neurones suddenly diminish in size to form, in rostral order, the almost coextensive caudal and oral pontine tegmental reticular nuclei. It then expands into the cuneiform nucleus and subcuneiform nucleus, before fading away in the midbrain tegmentum.
Axons of medial reticular column neurones form a multisynaptic ascending and descending system within the column, and ultimately enter the spinal cord and diencephalon. Descending fibres form the pontospinal (lateral reticulospinal), and bulbospinal (medial reticulospinal) tracts. Pontospinal axons arise from neurones in the caudal and oral parts of the pontine reticular nucleus, descend uncrossed in the ventral spinal funiculus, and terminate in spinal cord laminae VII, VIII and IX. Bulbospinal axons descend bilaterally to end in laminae VII, VIII, IX, and X, and ipsilaterally to end in laminae IV, V and VI. The system modulates spinal motor function and segmental nociceptive input.
Afferent components to the medial reticular nuclear column include the spinoreticular projection and collaterals of centrally projecting spinal trigeminal, vestibular and cochlear fibres. Spinoreticular fibres arise from neurones in the intermediate grey matter of the spinal cord. They decussate in the ventral white commissure, ascend in the ventrolateral funiculus, usually via several neurones, and terminate not only at all levels of the medial column of reticular nuclei but also in the intralaminar nuclei of the thalamus. Three areas of the medial reticular zone receive particularly high densities of terminations. These are the combined caudal and rostral ends of the gigantocellular and central nuclei respectively; the caudal pontine reticular nucleus; and the pontine tegmentum. Retinotectal and tectoreticular fibres relay visual information and the medial forebrain bundle transmits olfactory impulses.
Efferents from the medial column of reticular nuclei project through a multisynaptic pathway within the column to the thalamus. Areas of maximal termination of spinoreticular fibres also project directly to the intralaminar thalamic nuclei. The multisynaptic pathway is integrated into the lateral column of reticular nuclei with cholinergic neurones in the lateral pontine tegmentum. The intralaminar thalamic nuclei project directly to the striatum and neocortex.
LATERAL COLUMN OF RETICULAR NUCLEI
The lateral column of reticular nuclei contains six nuclear groups. These are the parvocellular reticular area; superficial ventrolateral reticular area; lateral pontine tegmental noradrenergic cell groups A1, A2, A4-A7 (A3 is absent in primates); adrenergic cell groups C1-C2; and cholinergic cell groups Ch5-Ch6. The column descends through the lower two-thirds of the lateral pontine tegmentum and upper medulla, where it lies between the gigantocellular nucleus medially, and the sensory trigeminal nuclei laterally. It continues caudally, and expands to form most of the reticular formation lateral to the raphe nuclei. It abuts the superficial ventrolateral reticular area, nucleus solitarius, nucleus ambiguus and vagal nucleus, and there contains the adrenergic cell group C2, and the noradrenergic group A2.The lateral paragigantocellular nucleus lies at the rostral pole of the diffuse superficial ventrolateral reticular area (at the level of the facial nucleus). The zone extends caudally as the nucleus retroambiguus and descends into the spinal cord. It contains noradrenergic cell groups A1, A2, A4 and A5, and the adrenergic cell group C1. The ventrolateral reticular area is involved in cardiovascular, respiratory, vasoreceptor and chemoreceptor reflexes, and in the modulation of nociception. The A2 or noradrenergic dorsal medullary cell group lies in the nucleus of the tractus solitarius, vagal nucleus and adjoining parvocellular reticular area. Adrenergic group C1 lies rostral to the A2 cell group. Noradrenergic cell group A4 extends into the lateral pontine tegmentum, along the subependymal surface of the superior cerebellar peduncle. Noradrenergic group A5 forms part of the paragigantocellular nucleus in the caudolateral pontine tegmentum. Noradrenergic cell group A5 and adrenergic cell group C1 probably function as centres of vasomotor control. The entire region is subdivided into functional areas on the basis of stimulation experiments in animals, in which vasoconstrictor, cardioaccelerator, depressor, inspiratory, expiratory and sudomotor effects have been elicited.
The lateral pontine tegmental reticular grey matter is related to the superior cerebellar peduncle and forms the medial and lateral parabrachial nuclei and the ventral Kölliker-Fuse nucleus, a pneumotaxic centre. The locus coeruleus (noradrenergic cell group A6), area subcoeruleus, noradrenergic cell group A7, and cholinergic group Ch5 in the pedunculopontine tegmental nucleus, are all located in the lateral pontine and mesencephalic tegmental reticular zones. The mesencephalic group Ch5 is continuous caudally with cell group Ch6 in the pontine central grey matter.
Cell group A6 contains all the noradrenergic cells in the central region of the locus coeruleus. Group A6 has ventral (nucleus subcoeruleus, A6 Sc), rostral and caudolateral extensions, the latter merges with the A4 group. The locus coeruleus probably functions as an attention centre, focusing neural functions to prevailing needs. The noradrenergic A7 group occupies the rostroventral part of the pontine tegmentum and is continuous with groups A5 and A1 through the lateral rhombencephalic tegmentum. The A7, A5, A1 complex is also connected by noradrenergic cell clusters with group A2, caudally, and group A6, rostrally. The A5 and A7 groups lie mainly within the medial parabrachial and Kölliker-Fuse nuclei. Reticular neurones in the lateral pontine tegmental reticular area, like those of the ventrolateral zone, function to regulate respiratory, cardiovascular and gastrointestinal activity. Two micturition centres are located in the dorsomedial and ventrolateral parts of the lateral pontine tegmentum.
The connections of the lateral column reticular nuclei are complex. The short ascending and descending axons of the parvocellular reticular area constitute bulbar reflex pathways, which connect all branchiomotor nuclei and the hypoglossal nucleus with central afferent cranial nerve complexes through a propriobulbar system. The area also receives descending afferents from the contralateral motor cortex via the corticotegmental tract, and from the contralateral red nucleus via the rubrospinal tract. The longitudinal catacholamine bundle passes through the parvocellular reticular formation.
The superficial ventrolateral reticular area receives some input from the spinal cord, insular cortex and amygdala, but the principal projection is from the nucleus solitarius and subserves cardiovascular, baroreceptor, chemoreceptor and respiratory reflexes. Reticulospinal afferents from the region terminate bilaterally on sympathetic preganglionic neurones in the thoracic spinal cord. Afferents from the pneumotaxic centre project to an inspiratory centre in the ventrolateral part of the nucleus solitarius, and a mixed expiratory-inspiratory centre in the superficial ventrolateral reticular area. Inspiratory neurones in both centres monosynaptically project to the phrenic and intercostal motor neurones. Axons of expiratory neurones terminate on lower motor neurones which innervate intercostal and abdominal musculature.
The superficial ventrolateral area is also the seat of the visceral alerting response. Fibres from the hypothalamus, periaqueductal grey matter and midbrain tegmentum mediate increased respiratory activity, raised blood pressure, tachycardia, vasodilation in skeletal muscle and renal and gastrointestinal vasoconstriction. Ascending efferents from the superficial ventrolateral area synapse on neurones of the supraoptic and paraventricular hypothalamic nuclei. Excitation of these neurones causes release of vasopressin from the neurohypophysis. Medullary noradrenergic cell groups A1 and A2 also innervate (directly and indirectly) the median eminence, and control the release of growth hormone, luteinizing hormone and adrenocorticotrophic hormone (ACTH).
The lateral pontine tegmentum, particularly the parabrachial region, is reciprocally connected to the insular cortex. It shares reciprocal projections with the amygdala through the ventral amygdalofugal pathway, medial forebrain bundle and central tegmental tract, and with the hypothalamic, median preoptic and paraventricular nuclei, which preferentially project to the lateral parabrachial nucleus and the micturition centres. It also shares reciprocal bulbar projections, many from the pneumotaxic centre, with the nucleus solitarius and superficial ventrolateral reticular area.
Reticulospinal fibres descend from the lateral pontine tegmentum. A mainly ipsilateral subcoeruleospinal pathway is distributed to all spinal segments of the cord through the lateral spinal funiculus. Crossed pontospinal fibres descend from the ventrolateral pontine tegmentum, decussate in the rostral pons and occupy the contralateral dorsolateral spinal funiculus. They terminate in laminae I, II, V and VI of all spinal segments of the cord. Fibres from the pneumotaxic centre innervate the phrenic nucleus and T1-T3 sympathetic preganglionic neurones bilaterally through this projection system.
Bilateral projections from the micturition centres travel in the lateral spinal funiculus. They terminate on preganglionic parasympathetic neurones in the sacral cord (which innervate the detrusor muscle in the urinary bladder), and on neurones in the nucleus of Onuf (which innervate the musculature of the pelvic floor and the anal and urethral sphincters).
Descending fibres of the A6 noradrenergic neurones of the locus coeruleus project into the longitudinal dorsal fasciculus (as the caudal limb of the dorsal periventricular pathway), and into the caudal limb of the dorsal noradrenergic bundle (as part of the longitudinal catecholamine bundle). In this way they innervate, mainly ipsilaterally, all other rhombencephalic reticular areas, principal and spinal trigeminal nuclei, pontine nuclei, cochlear nuclei, nuclei of the lateral lemniscus, and, bilaterally, all spinal preganglionic autonomic neurones and the ventral region of the dorsal horn in all segments of the spinal cord. Other axons that contribute to the longitudinal catecholamine bundle originate from cell groups C1, A1, A2, A5 and A7. The main projection is a descending one from cell groups C1 and A5, which are sudomotor neural control centres and innervate preganglionic sympathetic neurones.
Most ascending fibres from the locus coeruleus pass in the dorsal noradrenergic (or tegmental) bundle; others run in either the rostral limb of the dorsal periventricular pathway or in the superior cerebellar peduncle. The latter fibres terminate on the deep cerebellar nuclei. The dorsal noradrenergic bundle is large and runs through the ventrolateral periaqueductal grey matter to join the medial forebrain bundle in the hypothalamus, from where fibres continue forward to innervate all rostral areas of the brain. The pathway contains efferent and afferent axons that reciprocally connect the locus coeruleus with adjacent structures along its course, e.g. central mesencephalic grey matter, dorsal raphe nucleus, superior and inferior colliculi, interpeduncular nucleus, epithalamus, dorsal thalamus, habenular nuclei, amygdala, septum, olfactory bulb and anterior olfactory nucleus, entire hippocampal formation and neocortex. Fibres from the locus coeruleus which travel in the rostral limb of the dorsal periventricular pathway, ascend in the ventromedial periaqueductal grey matter adjacent to the longitudinal dorsal fasciculus and terminate in the parvocellular part of the paraventricular nucleus in the hypothalamus.
The functions of the locus coeruleus and related tegmental noradrenergic cell groups are poorly understood, largely because the afferent neurones that drive them have yet to be identified. The diversity of their rostral and caudal projections suggests a holistic role in central processing. In animals, firing rates of locus coeruleus neurones peak during wakefulness and decrease during sleep - they cease almost completely during rapid eye movement (REM) sleep. During wakefulness, firing rates are augmented when novel stimuli are presented. The locus coeruleus may, therefore, function to control the level of attentiveness. Other functions that have been ascribed to the locus coeruleus include control of the wake-sleep cycle, regulation of blood flow, and maintenance of synaptic plasticity.
The A1, A2, A5 and A7 noradrenergic cell groups project rostrally, mainly through the central tegmental tract. Their axons constitute a major longitudinal catecholamine pathway that continues through the medial forebrain bundle and ends in the amygdala, lateral septal nucleus, bed nucleus of the stria terminalis, nucleus of the diagonal band and the hypothalamus. The ascending dorsal periventricular pathway contains a few non-coerulean noradrenergic fibres, which terminate in the periventricular region of the thalamus.
Propriobulbar projections receive a contribution from the diffusely organized dorsal medullary and lateral tegmental noradrenergic cell groups. These interconnect cranial nerve nuclei and other reticular cell groups, particularly those of the vagus, facial and trigeminal nerves, and the rhombencephalic raphe and parabrachial nuclei.
Three precerebellar nuclei, the lateral and paramedian reticular nuclei and the nucleus of the pontine tegmentum, are involved in the relay of spinal information into the vermis and paravermal regions of the ipsilateral cerebellar hemisphere. They receive inputs from the contralateral primary motor and sensory neocortices, and the ipsilateral cerebellar and vestibular nuclei and spinal cord (the latter through the ascending spinoreticular pathway). This system augments the dorsal and ventral spinocerebellar, cuneocerebellar, accessory cuneocerebellar and trigeminocerebellar tracts.